Invima issues urgent health alert for counterfeit antivenom: how to differentiate it

The National Institute for Food and Drug Surveillance (Invima) issued an urgent health alert on Wednesday, May 28, 2025, after confirming the circulation of counterfeit units of polyvalent antivenom , a vital medication in the treatment of venomous snakebites.
The warning was prompted by a complaint from the National Institute of Health (INS), the entity that holds health registration 2019M-0013350-R1 and is responsible for distributing the product. According to the INS, suspicious bottles were identified that do not correspond to the original authorized product.
Currently, this legitimate serum is not available for commercial sale, as its distribution is restricted exclusively to areas of the country with a high risk of snakebite accidents, according to priorities established by the INS.
How to differentiate a counterfeit medicine from an original one? After a technical analysis, Invima's Technical Coordination and Support Group detected notable differences between the authentic product and the counterfeit bottles, such as the color of the container, the type of cap, and the lack of traceability with the manufacturer or official distributors. Due to these irregularities, and in accordance with Decree 677 of 1995, Invima classified the product as fraudulent.
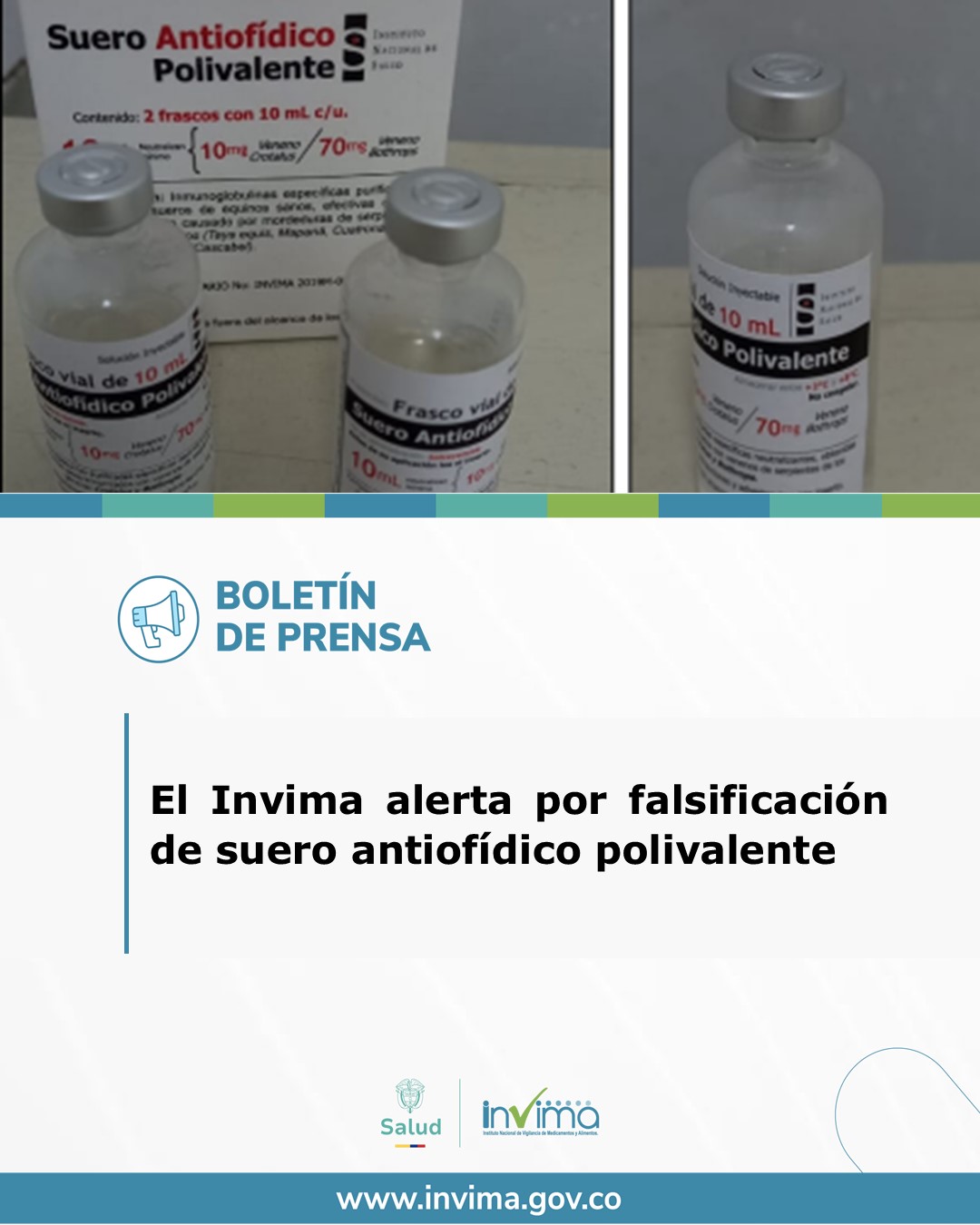
Polyvalent antivenom is being counterfeited, warns INVIMA. Photo: INVIMA
The agency warned that the use of these counterfeit serums poses a serious risk to public health. The lack of quality, safety, or efficacy guarantees, and the lack of known contents and storage conditions, could cause severe adverse effects in patients requiring urgent medical attention.
Recommendations to citizens, according to INVIMA • Always check the health registration number at www.invima.gov.co
• If you have purchased or are using the product, immediately discontinue use and inform Invima or local authorities.
• Report any adverse events to the email: [email protected]
Invima also warned about the illegal sale of a supplement without health registration and reiterated its call to the public, healthcare professionals, EPS, IPS, and regional entities to always verify the authenticity of medications through the official website.
Finally, he requested that the distribution of antivenom not originating from official channels be refrained from, and that inspection, surveillance, and control measures be strengthened. If counterfeit products are detected, they must be confiscated and destroyed following established protocols.
LATEST NEWS EDITORIAL
eltiempo

%3Aformat(jpg)%3Aquality(99)%3Awatermark(f.elconfidencial.com%2Ffile%2Fa73%2Ff85%2Fd17%2Fa73f85d17f0b2300eddff0d114d4ab10.png%2C0%2C275%2C1)%2Ff.elconfidencial.com%2Foriginal%2F549%2F6f5%2Fb55%2F5496f5b55708098204036b008b4f46cf.jpg&w=3840&q=100)
%3Aformat(jpg)%3Aquality(99)%3Awatermark(f.elconfidencial.com%2Ffile%2Fbae%2Feea%2Ffde%2Fbaeeeafde1b3229287b0c008f7602058.png%2C0%2C275%2C1)%2Ff.elconfidencial.com%2Foriginal%2F830%2Fef4%2Fb19%2F830ef4b19d68f9b63f1e4b8dc9b6134b.jpg&w=3840&q=100)
%3Aformat(jpg)%3Aquality(99)%3Awatermark(f.elconfidencial.com%2Ffile%2Fbae%2Feea%2Ffde%2Fbaeeeafde1b3229287b0c008f7602058.png%2C0%2C275%2C1)%2Ff.elconfidencial.com%2Foriginal%2Fd2c%2F183%2F066%2Fd2c18306627dc91ef477ac805d14c20f.jpg&w=3840&q=100)

